Abstract
Introduction: Veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a potentially life-threatening complication of hematopoietic cell transplantation (HCT) conditioning that may also develop after high-dose chemotherapy. Multiorgan failure (MOF) is associated with the most severe form of VOD/SOS and, if untreated, may result in a mortality rate of >80%. Defibrotide is approved for the treatment of hepatic VOD/SOS with renal or pulmonary dysfunction post-HCT in the US and for severe hepatic VOD/SOS post-HCT in patients aged >1 month (mo) in the EU. The DEFIFrance study collected real-world data on the efficacy and safety of defibrotide from HCT centers in France. This analysis presents final, long-term data on the primary study population: patients who received defibrotide treatment for severe/very severe VOD/SOS post-HCT.
Methods: This post-marketing registry study collected retrospective and prospective real-world data on patients receiving defibrotide at 53 HCT centers in France. Diagnosis of VOD/SOS was at the investigator's discretion using standard criteria, per typical clinical practice. Disease severity was categorized using adult EBMT severity criteria in patients aged ≥18 years (y), and patients aged <18 y were retrospectively/prospectively categorized using pediatric EBMT severity criteria. The primary endpoints were Day 100 post-HCT survival and complete response (CR; total serum bilirubin <2 mg/dL and MOF resolution per investigators' assessment) in patients with severe/very severe VOD/SOS. A secondary endpoint was the evaluation of treatment-emergent serious adverse events (SAEs) of interest: hemorrhage, coagulopathy, injection-site reactions, infections, and thromboembolic events, irrespective of relationship to treatment.
Results: Of 798 defibrotide-treated patients enrolled in the study, 251 patients received defibrotide for the treatment of severe/very severe VOD/SOS post-HCT (severe: 117 [47%]; very severe: 134 [53%]). Median age was 45 y (range: 0, 74) and 58 (23%) patients were <18 y of age. The most common primary diagnoses were acute myeloid leukemia (68 [27%]) and acute lymphoblastic leukemia (49 [20%]). Common (>50%) risk factors included prior treatment with hepatotoxic drugs (150 [60%]), iron overload (133 [58%]), and relapsed/refractory disease (137 [55%]). Risk factors of interest included prior HCT (29/239 [12%]), and prior treatment with gemtuzumab ozogamicin (17/251 [7%]) and inotuzumab ozogamicin (6/251 [2%]). At diagnosis, 55/250 (22%) patients had anicteric VOD/SOS (bilirubin levels ≤2 mg/dL). Median (range) time from VOD/SOS diagnosis to defibrotide administration was 0 (-1, 24) days.
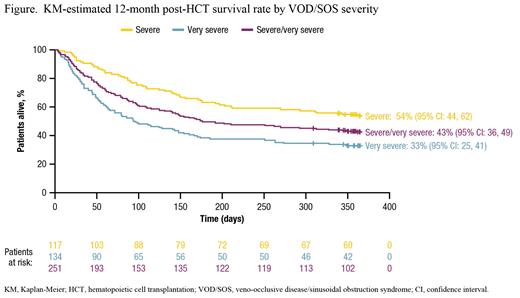
The Kaplan-Meier (KM)-estimated Day 100 post-HCT survival rate was 61% (95% confidence interval [CI]: 55%, 67%) in patients with severe/very severe VOD/SOS, with a higher survival rate seen in patients with severe versus very severe disease. The post-HCT survival rate in patients with severe/very severe VOD/SOS was 50% at 6 mo and 43% at 12 mo (Figure). KM-estimated survival rates at 6 and 12 mo were also higher for those with severe versus very severe disease (Figure). The KM-estimated CR rate by Day 100 among patients with severe/very severe VOD/SOS post-HCT was 74% (95% CI: 66%, 81%). Similar to the pattern observed for the survival rate, a higher CR rate was observed by Day 100 in patients with severe (84% [95% CI: 74, 92]) versus very severe (63% [95% CI: 52, 74]) VOD/SOS.
Treatment-emergent SAEs of interest occurred in 29% of patients with severe/very severe VOD/SOS post-HCT; the most common (≥2%) treatment-emergent SAE categories were infection (17%), hemorrhage (16%), and hypotension (2%). Mortality due to VOD/SOS at 12 mo was 15%.
Conclusions: The DEFIFrance study represents the largest collection of real-world data on post-registration use of defibrotide. The efficacy and safety observed in this study add to evidence from prior studies supporting the utility of defibrotide for treating patients with severe/very severe VOD/SOS post-HCT in a real-world setting. Among patients receiving defibrotide for VOD/SOS post-HCT, outcomes were better in patients with severe versus very severe VOD/SOS, highlighting the importance of early VOD/SOS diagnosis and treatment, before patients reach the most severe stage of VOD/SOS.
Mohty: Sanofi: Honoraria, Research Funding; Pfizer: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Jazz: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria; Celgene: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Astellas: Honoraria; Amgen: Honoraria; Adaptive Biotechnologies: Honoraria. Blaise: Jazz Pharmaceuticals: Honoraria. Peffault De Latour: Amgen: Consultancy, Other, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Jazz Pharmaceuticals: Honoraria. Labopin: Jazz Pharmaceuticals: Honoraria. Detrait: Jazz Pharmaceuticals: Research Funding. Huynh: Jazz Pharmaceuticals: Honoraria. Renard: Jazz Pharmaceuticals: Research Funding. Asubonteng: Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Delaval: Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Yakoub-Agha: Jazz Pharmaceuticals: Honoraria. Dalle: Jazz Pharmaceuticals: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal